Specific heat for some common liquids and fluids - acetone, oil, paraffin, water and many more
SODIUM HYPOCHLORITE SHC. 9.10 Vapor (Gas) Specific Gravity: Not pertinent 9.11 Ratio of Specific Heats of Vapor (Gas): Not pertinent. LIQUID HEAT CAPACITY Temperature (degrees F) British thermal unit per pound-F 42 44 46 48 50 52 54 56 58 60 62 64 66 68 70 72 74 76 0.908 0.908 0.908 0.908 0.908. Caustic soda (sodium hydroxide or NaOH) is most commonly manufactured by the electrolysis of a sodium chloride (NaCl) solution. OxyChem manufactures caustic soda using either membrane or diaphragm electrolytic cells. OxyChem does not use mercury based electrolytic cells to produce caustic soda.
The specific heat for some commonly used liquids and fluids is given in the table below.
For conversion of units, use the Specific heat online unit converter.
See also tabulated values of specific heat of gases, food and foodstuff, metals and semimetals, common solids and other common substances as well as values of molar specific heat of common organic substances and inorganic substances.
| Product | Specific Heat - cp - | |
|---|---|---|
| (kJ/(kg K)) | (Btu/(lb oF)) (Kcal/kg oC) | |
| Acetic acid | 2.043 | 0.49 |
| Acetone | 2.15 | 0.51 |
| Alcohol, ethyl 32oF (ethanol) | 2.3 | 0.548 |
| Alcohol, ethyl 104oF (ethanol) | 2.72 | 0.65 |
| Alcohol, methyl. 40 - 50oF | 2.47 | 0.59 |
| Alcohol, methyl. 60 - 70oF | 2.51 | 0.6 |
| Alcohol, propyl | 2.37 | 0.57 |
| Ammonia, 32oF | 4.6 | 1.1 |
| Ammonia, 104oF | 4.86 | 1.16 |
| Ammonia, 176oF | 5.4 | 1.29 |
| Ammonia, 212oF | 6.2 | 1.48 |
| Ammonia, 238oF | 6.74 | 1.61 |
| Aniline | 2.18 | 0.514 |
| Benzene, 60oF | 1.8 | 0.43 |
| Benzene, 150oF | 1.92 | 0.46 |
| Benzine | 2.1 | |
| Benzol | 1.8 | 0.43 |
| Bismuth, 800oF | 0.15 | 0.0345 |
| Bismuth, 1000oF | 0.155 | 0.0369 |
| Bismuth, 1400oF | 0.165 | 0.0393 |
| Bromine | 0.47 | 0.11 |
| n-Butane, 32oF | 2.3 | 0.55 |
| Calcium Chloride | 3.06 | 0.73 |
| Carbon Disulfide | 0.992 | 0.237 |
| Carbon Tetrachloride | 0.866 | 0.207 |
| Castor Oil | 1.8 | 0.43 |
| Chloroform | 1.05 | 0.251 |
| Citron Oil | 1.84 | 0.44 |
| Decane | 2.21 | 0.528 |
| Diphenylamine | 1.93 | 0.46 |
| Dodecane | 2.21 | 0.528 |
| Dowtherm | 1.55 | 0.37 |
| Ether | 2.21 | 0.528 |
| Ethyl ether | 2.22 | 0.529 |
| Ethylene glycol | 2.36 | 0.56 |
| Dichlorodifluoromethane R-12 saturated -40oF | 0.88 | 0.211 |
| Dichlorodifluoromethane R-12 saturated 0oF | 0.91 | 0.217 |
| Dichlorodifluoromethane R-12 saturated 120oF | 1.02 | 0.244 |
| Fuel Oil min. | 1.67 | 0.4 |
| Fuel Oil max. | 2.09 | 0.5 |
| Gasoline | 2.22 | 0.53 |
| Glycerine | 2.43 | 0.576 |
| Heptane | 2.24 | 0.535 |
| Hexane | 2.26 | 0.54 |
| Hydrochlor acid | 3.14 | |
| Iodine | 2.15 | 0.51 |
| Kerosene | 2.01 | 0.48 |
| Linseed Oil | 1.84 | 0.44 |
| Light Oil, 60oF | 1.8 | 0.43 |
| Light Oil, 300oF | 2.3 | 0.54 |
| Mercury | 0.14 | 0.03 |
| Methyl alcohol | 2.51 | |
| Milk | 3.93 | 0.94 |
| Naphthalene | 1.72 | 0.41 |
| Nitric acid | 1.72 | |
| Nitro benzole | 1.52 | 0.362 |
| Octane | 2.15 | 0.51 |
| Oil, Castor | 1.97 | 0.47 |
| Oil, Olive | 1.97 | 0.47 |
| Oil, mineral | 1.67 | 0.4 |
| Oil, turpentine | 1.8 | |
| Oil, vegetable | 1.67 | 0.4 |
| Olive oil | 1.97 | 0.47 |
| Paraffin | 2.13 | 0.51 |
| Perchlor ethylene | 0.905 | |
| Petroleum | 2.13 | 0.51 |
| Petroleum ether | 1.76 | |
| Phenol | 1.43 | 0.34 |
| Potassium hydrate | 3.68 | 0.88 |
| Propane, 32oF | 2.4 | 0.576 |
| Propylene | 2.85 | 0.68 |
| Propylene Glycol | 2.5 | 0.60 |
| Sesame oil | 1.63 | 0.39 |
| Sodium, 200oF | 1.38 | 0.33 |
| Sodium, 1000oF | 1.26 | 0.3 |
| Sodium hydrate | 3.93 | 0.94 |
| Soya bean oil | 1.97 | 0.47 |
| Sulfuric acid concentrated | 1.38 | |
| Sulfuric acid | 1.34 | |
| Toluene | 1.72 | 0.41 |
| Trichlor ethylene | 1.30 | |
| Tuluol | 1.51 | 0.36 |
| Turpentine | 1.72 | 0.411 |
| Water, fresh | 4.19 | 1 |
| Water, sea 36oF | 3.93 | 0.938 |
| Xylene | 1.72 | 0.41 |
- 1 kJ/(kg K) = 1000 J/(kgoC) = 0.2389 kcal/(kg oC) = 0.2389 Btu/(lbmoF)
- T(oC) = 5/9[T(oF) - 32]
For conversion of units, use the Specific heat online unit converter.
See also tabulated values of specific heat of Gases, Food and foodstuff, Metals and semimetals, Common solids and other Common substances.
Heating Energy
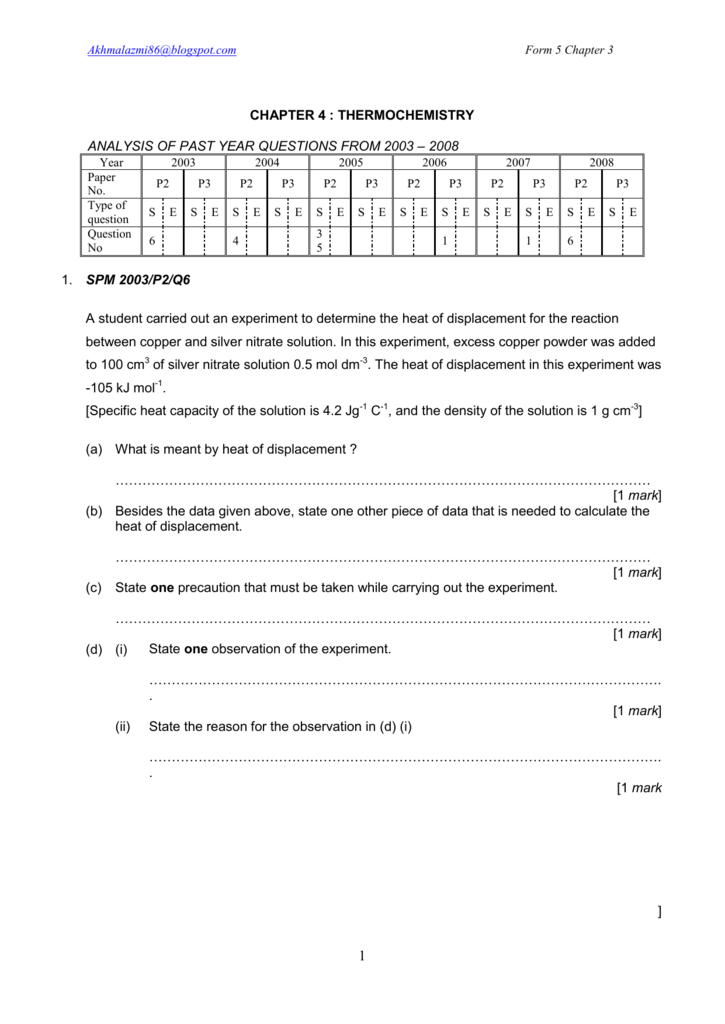
The energy required to heat a product can be calculated as
q = cp m dt (1)
where
q = heat required (kJ)
cp = specific heat (kJ/kg K, kJ/kg oC)
dt = temperature difference (K, oC)
Sodium Hydroxide Solution Specific Heat Capacity
Example - Required Heat to increase Temperature i Water
10 kg of water is heated from 20 oC to 100 oC - a temperature difference 80 oC (K). The heat required can be calculated as
q = (4.19 kJ/kg K) (10 kg) (80 oC)
Sendtox mac free download. = 3352 kJ
Related Topics
- Material Properties - Material properties for gases, fluids and solids - densities, specific heats, viscosities and more
- Thermodynamics - Effects of work, heat and energy on systems
Related Documents
- Ammonia - Density at Varying Temperature and Pressure - Online calculator, figures and tables showing density and specific weight of ammonia at temperatures ranging -50 to 425 °C (-50 to 800 °F) at atmospheric and higher pressure - Imperial and SI Units
- Ammonia - Dynamic and Kinematic Viscosity - Online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging from -73 to 425°C (-100 to 800°F) at pressure ranging from 1 to 1000 bara (14.5 - 14500 psia) - SI and Imperial Units
- Ammonia - Properties at Gas-Liquid Equilibrium Conditions - Figures and tables showing how the properties of liquid and gaseous ammonia changes along the boiling/condensation curve (temperature and pressure between triple point and critical point conditions). An ammonia phase diagram are included.
- Ammonia - Specific Heat at varying Temperature and Pressure - Online calculator, figures and tables showing specific heat, CP and CV, of gasous and liquid ammonia at temperatures ranging from -73 to 425°C (-100 to 800°F) at pressure ranging from 1 to 100 bara (14.5 - 1450 psia) - SI and Imperial Units
- Ammonia - Thermal Conductivity at Varying Temperature and Pressure - Online calculator, figures and tables showing thermal conductivity of liquid and gaseous ammonia at temperatures ranging -70 to 425 °C (-100 to 800 °F) at atmospheric and higher pressure - Imperial and SI Units
- Ammonia - Thermophysical Properties - Chemical, Physical and Thermal Properties of Ammonia. Phase diagram included.
- Ammonia - Vapour Pressure at gas-liquid equilibrium - Figures and table showing ammonia saturation pressure at boiling point, SI and Imperial units
- Electric Heating of a Mass - Electric heating of an object or mass - energy supply and temperature change
- Heat Capacity - The heat capacity of a substance is the amount of heat required to change its temperature by one degree, and has units of energy per degree
- Heat Emission from Pipes Submerged in Oil or Fat - Heat emission from steam or water heating pipes submerged in oil or fat - forced and natural circulation
- Heat Up Applications - Energy Required and Heat Transfer Rates - Energy required to heat up a substance
- Light Oil Suction Flow Velocity - Recommended suction flow velocity when pumping light oils
- Liquid ammonia - Thermal Properties at saturation pressure - Density, specific heat, thermal conductivity, viscosity and Prandtls no. of liquid ammonia at its saturation pressure
- Mixing Fluids - Final mass and temperature when mixing fluids
- Mixing Liquids and/or Solids - Final Temperatures - Calculate the final temperature when liquids or solids are mixed
- Oil Tanks Heat Loss - Heat loss from lagged and unlagged, sheltered and exposed oil tanks
- Polymers - Specific Heats - Specific heat of polymers like epoxy, PET, polycarbonate and more
- Specific Heat - Online Unit Converter - Online specific heat converter with the most commonly used units
- Specific Heat and Individual Gas Constants of Gases - Specific heat at constant volume, specific heat at constant pressure, specific heat ratio and individual gas constant - R - common gases as argon, air, ether, nitrogen and many more .
- Specific Heat of Food and Foodstuff - Specific heat of common food and foodstuff like apples, bass, beef, pork and many more
- Specific Heat of Solids - Common solids - like brick, cement, glass and many more - and their specific heats - in Imperial and SI units
- Steel Pipes and Temperature Expansion - Temperature expansion of carbon steel pipes
- Storing Thermal Heat in Materials - Energy stored as sensible heat in materials
- Sulfuric Acid - Density - Density of sulfuric acid at various temperatures and concentrations
- Water - Specific Heat - Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 °C (32-700 °F) - SI and Imperial units
Tag Search
- en: specific heat capacity fluids liquids
- es: fluidos capacidad calorífica líquidos específicos
- de: spezifische Wärmekapazität Flüssigkeiten Flüssigkeiten
- Formula: HNaO
- Molecular weight: 39.9971
- IUPAC Standard InChI:
- InChI=1S/Na.H2O/h;1H2/q+1;/p-1
- Download the identifier in a file.
- IUPAC Standard InChIKey:HEMHJVSKTPXQMS-UHFFFAOYSA-M
- CAS Registry Number: 1310-73-2
- Chemical structure:
This structure is also available as a 2d Mol fileor as a computed3d SD file
The 3d structure may be viewed usingJavaorJavascript. - Species with the same structure:
- Information on this page:
- Other data available:
- Data at other public NIST sites:
- Options:


Data at NIST subscription sites:
NIST subscription sites provide data under theNIST Standard ReferenceData Program, but require an annual fee to access.The purpose of the fee is to recover costs associatedwith the development of data collections included insuch sites. Your institution may already be a subscriber.Follow the links above to find out more about the datain these sites and their terms of usage.
Solid Phase Heat Capacity (Shomate Equation)
Go To:Top, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Cp° = A + B*t + C*t2 + D*t3 + E/t2
H° − H°298.15= A*t + B*t2/2 + C*t3/3 + D*t4/4 − E/t + F − H
S° = A*ln(t) + B*t + C*t2/2 + D*t3/3 − E/(2*t2) + G
Cp = heat capacity (J/mol*K)
H° = standard enthalpy (kJ/mol)
S° = standard entropy (J/mol*K)
t = temperature (K) / 1000.
View plotRequires a JavaScript / HTML 5 canvas capable browser.
| Temperature (K) | 298. - 572. | 572. - 596. |
|---|---|---|
| A | 419.4837 | 86.02304 |
| B | -1717.754 | 0.000000 |
| C | 2953.573 | 0.000000 |
| D | -1597.221 | 0.000000 |
| E | -6.046884 | 0.000000 |
| F | -517.8662 | -448.8512 |
| G | 933.0738 | 169.6281 |
| H | -425.9312 | -425.9312 |
| Reference | Chase, 1998 | Chase, 1998 |
| Comment | Data last reviewed in December, 1970 | Data last reviewed in December, 1970 |
| Temperature (K) | Cp (J/mol*K) | S° (J/mol*K) | -(G° - H°298.15)/T (J/mol*K) | H° - H°298.15 (kJ/mol) |
|---|---|---|---|---|
| 298. | 59.52 | 64.43 | 64.44 | -0.00 |
| 300. | 59.67 | 64.83 | 64.45 | 0.12 |
| 400. | 64.94 | 82.71 | 66.85 | 6.34 |
| 500. | 75.16 | 98.17 | 71.59 | 13.29 |
| Temperature (K) | Cp (J/mol*K) | S° (J/mol*K) | -(G° - H°298.15)/T (J/mol*K) | H° - H°298.15 (kJ/mol) |
|---|---|---|---|---|
| 572. | 86.02 | 121.6 | 75.62 | 26.29 |
References
Go To:Top, Solid Phase Heat Capacity (Shomate Equation), Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Chase, 1998
Chase, M.W., Jr.,NIST-JANAF Themochemical Tables, Fourth Edition,J. Phys. Chem. Ref. Data, Monograph 9, 1998, 1-1951. [all data]

Notes
Sodium Hydroxide Specific Heat Capacity Definition
Go To:Top, Solid Phase Heat Capacity (Shomate Equation), References
Concentrated Sodium Hydroxide
- Data from NIST Standard Reference Database 69:NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST)uses its best efforts to deliver a high quality copy of theDatabase and to verify that the data contained therein havebeen selected on the basis of sound scientific judgment.However, NIST makes no warranties to that effect, and NISTshall not be liable for any damage that may result fromerrors or omissions in the Database.
- Customer supportfor NIST Standard Reference Data products.